OUR SOLUTIONS
OUR SOLUTIONS
SonoCloud®
Unlocking and harnessing the efficacy of new and existing therapies to enhance treatment of brain diseases
 English
English
 OUR SOLUTIONS
OUR SOLUTIONSUnlocking and harnessing the efficacy of new and existing therapies to enhance treatment of brain diseases





 Carthera SonoCloud®
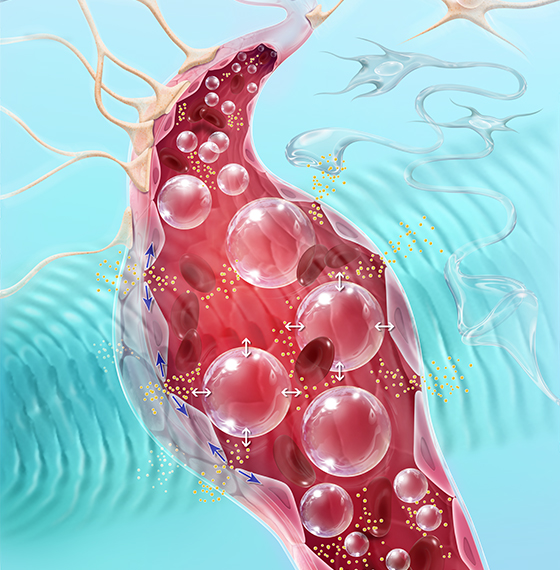
Carthera SonoCloud®The BBB blocks the penetration, and thus potential therapeutic effects, of over 95% of small molecule and 100% of large molecule drugs in the brain. SonoCloud® uses the therapeutic potential of pulsed ultrasound to temporarily disrupt the blood-brain barrier (BBB). By using SonoCloud®, the therapeutic efficacy of new and existing therapies can be unlocked and harnessed to improve the treatment of a wide range of brain diseases.
The SonoCloud® device is implanted in a skull window, below the skin, and is invisible. When activated for few minutes using a transdermal needle connection to an external control unit, the BBB is disrupted for several hours, leading to a window where drug therapies can be administered. By administering therapies when the BBB is disrupted, drugs can reach the brain in higher and more effective concentrations. This treatment can be repeated at each cycle of drug therapy.
Carthera® has developed several versions of SonoCloud®, including the SonoCloud-1 and SonoCloud-9 devices. These MRI-compatible devices are designed to disrupt large regions of the BBB to increase therapeutic efficacy of drugs in targeted brain regions and are currently in clinical trials for glioblastoma, brain metastases, and Alzheimer’s Disease. Carthera®’s patent portfolio provides strong protection for the company’s products.
 Carthera SonoCloud® Technology
Carthera SonoCloud® TechnologyFew minutes of ultrasound
Several hours of blood-brain barrier opening
Substantial increase of concentration of therapeutic molecules
Potential solutions to high clinical unmet needs
Low intensity pulsed ultrasound (LIPU) for blood-brain barrier disruption has been in pre-clinical development for over 20 years.
The technology relies on using low intensity ultrasound (similar to levels used in diagnostic imaging applications) in combination with intravenous injection of a microbubble agent to stimulate a therapeutic effect. The LIPU leads to vibration of injected microbubbles in brain micro vessels. This mechanical effect temporarily disrupts the tight junctions of the BBB, increases active transport across the BBB and downregulates active drug efflux transporters.
Hundreds of pre-clinical articles have demonstrated the therapeutic potential of this technology to deliver small and large molecule drug therapies to the brain.
In addition, use of this technique alone, without additional drug therapies, may lead to improvement of Alzheimer’s Disease pathology, currently under clinical investigation.